Food and Drug Administration (FDA)
See the following -
FDA Plays Chicken With Antibiotics: Newly Disclosed Documents Reveal Agency's "High Risk" Gamble With Human Health
Here’s some unfortunate, but not so surprising news: The Food and Drug Administration has allowed 30 potentially harmful antibiotic additives to remain approved for use in food animals (cows, pigs and chickens), even though the agency’s own scientists found them to pose a risk to human health or lack necessary data on safety. Read More »
- Login to post comments
FDA Scrutinizes Antibacterial Products For Hormonal Disruption, Bacterial Resistance
[...] The FDA has announced that it is formally reconsidering “antibacterial” soaps and other personal-care products, charging that the antibacterial ingredients confer no benefit over regular soap and water while carrying extra risks. Read More »
- Login to post comments
FDA Staffers Sue Agency Over Surveillance of Personal E-Mail
The Food and Drug Administration secretly monitored the personal e-mail of a group of its own scientists and doctors after they warned Congress that the agency was approving medical devices that they believed posed unacceptable risks to patients, government documents show.
- Login to post comments
FDA Starts Beta-testing 'The Most Advanced Bioinformatics Platform in the World'
 The FDA has started testing the precisionFDA platform it developed with DNAnexus. The closed beta test phase is the precursor to a more widespread rollout of the system, which the CEO of DNAnexus has described as being "the most advanced bioinformatics platform in the world." DNAnexus was enlisted by the FDA to help with the project in August...
The FDA has started testing the precisionFDA platform it developed with DNAnexus. The closed beta test phase is the precursor to a more widespread rollout of the system, which the CEO of DNAnexus has described as being "the most advanced bioinformatics platform in the world." DNAnexus was enlisted by the FDA to help with the project in August...
- Login to post comments
FDA Takes Significant Steps To Address Antimicrobial Resistance
The U.S. Food and Drug Administration today is implementing a plan to help phase out the use of medically important antimicrobials in food animals for food production purposes, such as to enhance growth or improve feed efficiency. The plan would also phase in veterinary oversight of the remaining appropriate therapeutic uses of such drugs. Read More »
- Login to post comments
FDA Targets Essentials Oils: Sees EOs As Threat To New Ebola
The FDA issued warning letters this week to the two largest distributors of essentials oils in the United Sates, Young Living and dōTERRA. The FDA is claiming that their products are being marketed as unapproved drugs...
- Login to post comments
FDA Tells Google-Backed 23andMe to Halt DNA Test Service
23andMe Inc., the Google Inc.-backed DNA analysis company co-founded by Anne Wojcicki, was told by U.S. regulators to halt sales of its main product because it’s being sold without “marketing clearance or approval.” Read More »
- Login to post comments
FDA Tells Hospitals to Ditch IV Pumps That Can Be Hacked Remotely
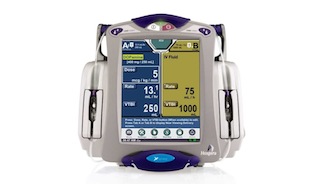 The Food and Drug Administration "strongly encourages" hospitals to stop using Hospira's Symbiq Infusion System, because it's vulnerable to cyberattacks that would allow a third party to remotely control dosages delivered via the computerized pumps. Unauthorized users are able to access the Symbiq system through connected hospital networks, according to the FDA and the Department of Homeland Security's Industrial Control Systems Cyber Emergency Response Team...
The Food and Drug Administration "strongly encourages" hospitals to stop using Hospira's Symbiq Infusion System, because it's vulnerable to cyberattacks that would allow a third party to remotely control dosages delivered via the computerized pumps. Unauthorized users are able to access the Symbiq system through connected hospital networks, according to the FDA and the Department of Homeland Security's Industrial Control Systems Cyber Emergency Response Team...
- Login to post comments
FDA to Advance Precision Medicine by Enabling Open Source Collaborative Informatics
 FDA plays an integral role in President Obama’s Precision Medicine Initiative, which foresees the day when an individual’s medical care will be tailored in part based on their unique characteristics and genetic make-up. Yet while more than 80 million genetic variants have been found in the human genome, we don’t understand the role that most of these variants play in health or disease. Achieving the President’s vision requires working collaboratively to ensure the accuracy of genetic tests in detecting and interpreting genetic variants. We are working towards that goal by developing an informatics community and supporting platform we call precisionFDA.
FDA plays an integral role in President Obama’s Precision Medicine Initiative, which foresees the day when an individual’s medical care will be tailored in part based on their unique characteristics and genetic make-up. Yet while more than 80 million genetic variants have been found in the human genome, we don’t understand the role that most of these variants play in health or disease. Achieving the President’s vision requires working collaboratively to ensure the accuracy of genetic tests in detecting and interpreting genetic variants. We are working towards that goal by developing an informatics community and supporting platform we call precisionFDA.
- Login to post comments
FDA Update On Animal Pharmaceutical Industry Response To Guidance #213
On December 11, 2013, the FDA announced the implementation of its plan to help phase out the use of medically important antimicrobials in food animals for food production purposes. [...] FDA asked affected sponsors to notify the agency in writing within three months, or by March 12, 2014, of their intent to engage with FDA as defined in Guidance for Industry (GFI) #213. Read More »
- Login to post comments
FDA Wants To Leverage Electronic Medical Records To Probe For Adverse Events
Keeping track of adverse events is a tricky task, even for regulators. Even when a drug has undergone a rigorous premarket assessment process, some risks may not become evident until a product is used by millions. And for other drugs, a particularly rare but serious side effect may take months, if not years, to be identified. Read More »
- Login to post comments
FDA Wants To Use EMRs To Streamline Adverse Drug Event Reporting
The U.S. Food and Drug Administration wants to leverage electronic medical records to probe for adverse drug events, according to a recent article in Regulatory Focus. Big data has the power to help expose adverse events more quickly than ever, and the FDA wants to use that to assess drug side effects. Read More »
- Login to post comments
FDA’s New Antibiotics Guidance Falls Short Of Improving Welfare And Reducing Health Risks From Factory Farming
The U.S. Food & Drug Administration’s release of its long-awaited policy is drawing criticism from a group of leading animal welfare organizations, including The ASPCA® (The American Society for the Prevention of Cruelty to Animals®), Farm Sanctuary, Animal Welfare Approved, and the Animal Legal Defense Fund. Read More »
- Login to post comments
FDA’s Public Data Project To Get An Upgrade Under $1.2M Contract
In an ongoing effort to improve openFDA, San Francisco-based Iodine will work on making the portal more user friendly...
- Login to post comments
FDA’s Step To Limit Animal Antibiotics Symbolic–Animal Husbandry Issues Must Still be Addressed
In 1977, the [FDA] let everyone know that there was strong evidence that the use of penicillin and tetracycline for anything other than treating disease in livestock, could lead to the development of super bugs strong enough to render the powerful antibiotics useless in people. [...] Now, [the FDA] has finally mustered the courage to approve a strongly worded recommendation for producers to stop using medically important antibiotics as growth promoters and to give veterinarians oversight over therapeutic uses of the life-saving drugs. Read More »
- Login to post comments