LabLynx Joins the COVID-19 Fight by Introducing Laboratory System Dedicated to Infectious Disease Diagnostic Testing
ATLANTA, April 9, 2020 /PRNewswire/ -- LabLynx, Inc., long-time leader in enterprise-level cloud-hosted LIMS/LIS, today announced it has developed the world's first LIMS (Laboratory Information Management System) dedicated specifically to COVID-19 diagnostic testing. Called "CovidLiMS," its main features include, crucially, swift setup-to-live time: 2-5 days, including training, according to LabLynx President John H. Jones.
 John H. JonesLabLynx, Inc has developed the world's first LIMS (Laboratory Information Management System) dedicated specifically to COVID-19 diagnostic testing called "CovidLiMS."
John H. JonesLabLynx, Inc has developed the world's first LIMS (Laboratory Information Management System) dedicated specifically to COVID-19 diagnostic testing called "CovidLiMS."
Jones said, "We have released it because we already had the platform in place, and so we used the platform to provide a low cost, turn-key LIMS for COVID-19 testing to address the demands of our existing clients and to address the real and urgent information management needed for the current crisis." Mr. Jones went on to say that "CovidLiMS addresses the needs of existing clients and of labs that need to perform COVID-19 testing but their existing LIMS is simply not set up to do it or they have no LIMS at all."
In fact, CovidLiMS is more than just a short-term solution to a single problem. "CovidLiMS is a platform for testing and managing data for all infectious diseases," said Jones. "This is being released to provide a quick, easy solution that can work without disrupting existing lab workflows, by having a LIMS devoted to infectious diseases that constitute a public health threat. We are making it so that both public and private health laboratories have a plug-and-play solution."
CovidLiMS, like LabLynx's flagship enterprise LIMS, is highly user-configurable and extensible, so that "while it may become adopted by labs to address this moment in time, it is actually a platform for comprehensive laboratory data management for the long term as well, with additional processes and tests - as well as departments and in fact other labs - able to be easily added," he explained.
CovidLiMS is designed to be used by labs regardless of whether they already have a LIMS/LIS or not. "LabLynx existing clients can have the CovidLiMS installed as a module to augment their existing LabLynx LIMS and other labs can add this LIMS to manage their COVID-19 or other infectious/pandemic disease data alongside their current system and go live in a matter of a few days and with low cost," said Jones.
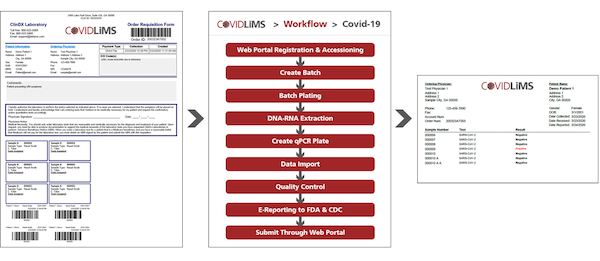 LIMS and COVID-19 Testing & Reporting
LIMS and COVID-19 Testing & Reporting
Contact LabLynx at www.lablynx.com or [email protected] / 866-LabLynx (522-5969) to get started free and to receive a free consultation on setting up your lab to perform COVID-19 and other infectious disease testing.
About LabLynx
LabLynx, Inc. was the first Laboratory Informatics provider to deliver a true browser-based Laboratory Information Management Solution in 1997. LabLynx, Inc. was incorporated as an independently owned and operated software company in July 2000.
LabLynx was once a product division of a diversified IT consulting company purchased by a group of investors experienced in both LIMS and Information Technology Management. The core LabLynx team has combined software development and laboratory management experience of over 575 human years invested in the LabLynx LIMS product lines.
Additionally, LabLynx has been the leader in establishing the paradigm of the LIMS as a platform-based, total laboratory solution, rather than just a means to track samples. The result is that LabLynx is a LIMS vendor dedicated to you, your information, and your workload management.
Media Contact:
John H. Jones
866-522-5969
SOURCE LabLynx, Inc.
Related Links
- Tags:
- cloud-hosted LIMS
- coronavirus
- COVID-19
- COVID-19 diagnostic testing
- COVID-19 testing
- CovidLiMS
- infectious disease data
- infectious diseases
- John H. Jones
- LabLynx
- laboratory data management
- laboratory information management system (LIMS)
- laboratory software
- private health laboratories
- public health laboratories
- public health threat
- Login to post comments