PraediTrial
 Identifying clinical trial sites that can fulfill subject recruitment goals can be a costly, time consuming, and inaccurate process. PraediTrial is a tool developed by Bitscopic that ensures a realistic assessment of trial sites’ subject population and capabilities to successfully conduct a clinical trial. In addition, PraediTrial stays with you through the recruitment cycle providing study coordinators with alerts to easily identify subjects for your trial.
Identifying clinical trial sites that can fulfill subject recruitment goals can be a costly, time consuming, and inaccurate process. PraediTrial is a tool developed by Bitscopic that ensures a realistic assessment of trial sites’ subject population and capabilities to successfully conduct a clinical trial. In addition, PraediTrial stays with you through the recruitment cycle providing study coordinators with alerts to easily identify subjects for your trial.
Conducting a clinical trial feasibility evaluation is one of the first crucial steps in successful clinical trial conduct. This demands a rigorous and realistic assessment of a site’s capability to conduct a clinical trial. Study sponsors and contract research organizations (CROs) distribute site selection questionnaires to collect information for evaluating whether to invite sites to participate in a study. Current questionnaires and the processes surrounding them are clearly inadequate since most sites underperform their contracted commitments, which are often scaled back from questionnaire estimates. For both sponsor and investigators, the ability to validate the potential enrollment pool for a study remains the most challenging pre-trial task.
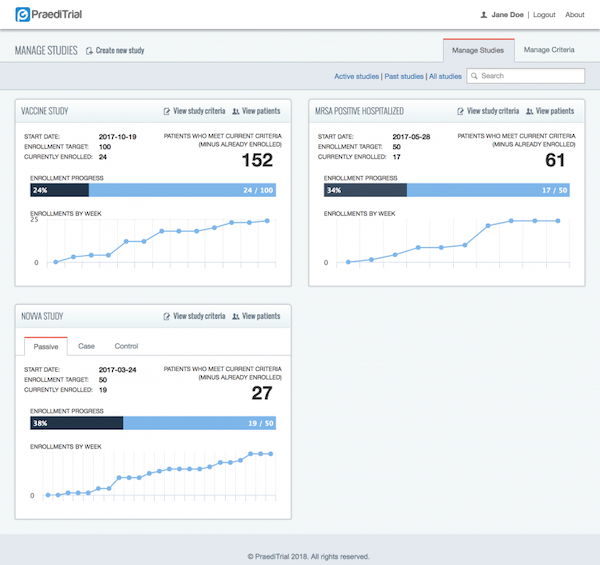
PraediTrial is designed to increase the accuracy of enrollment estimates, and assist the investigator throughout the recruitment process by identifying eligible study subjects. PraediTrial uses a studies’ inclusion/exclusion criteria to comb the electronic patient record for eligible study candidates. This increases enrollment prediction accuracy, and more importantly, decreases the burden of patient identification for the entire study team.
- Login to post comments