FDA data
See the following -
Soom Launches Mobile App That Notifies Patients, Caregivers and Nurses of Medical Device Recalls
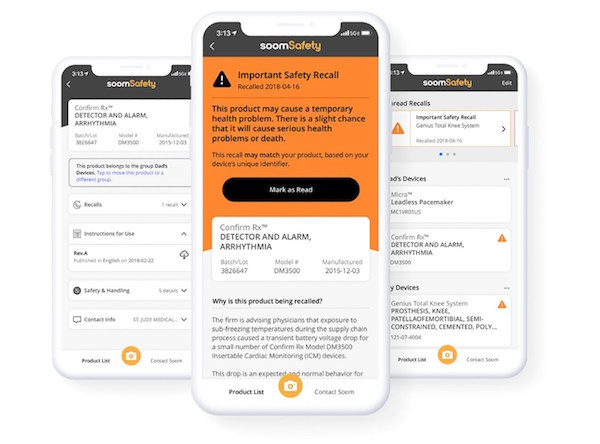 Soom, a pioneer in utilizing barcode and knowledge graph technologies to bridge information gaps between data sources and physical products, has introduced SoomSafety, an iOS mobile app that allows users to scan a medical device and receive instructions for use, safety and recall information directly from the device manufacturer and U.S. Food and Drug Administration (FDA). "We built SoomSafety to help patients and caregivers relying on implanted medical devices and using medical devices at home answer one critical question, 'Is this medical device safe to use?'" said Charlie Kim, President and CEO of Soom. "Our technology makes it possible to connect previously siloed medical device data, giving patients-and their caregivers-more proactive control over their health and safety."
Soom, a pioneer in utilizing barcode and knowledge graph technologies to bridge information gaps between data sources and physical products, has introduced SoomSafety, an iOS mobile app that allows users to scan a medical device and receive instructions for use, safety and recall information directly from the device manufacturer and U.S. Food and Drug Administration (FDA). "We built SoomSafety to help patients and caregivers relying on implanted medical devices and using medical devices at home answer one critical question, 'Is this medical device safe to use?'" said Charlie Kim, President and CEO of Soom. "Our technology makes it possible to connect previously siloed medical device data, giving patients-and their caregivers-more proactive control over their health and safety."
- Login to post comments